The only regulatory platform purpose-built for speed, compliance, and AI-powered review. From dossier assembly to agency submission — one unified ecosystem for your entire regulatory operation.
Request a Demo
Enterprise-Grade Security & Compliance
Integrates With Your Existing Tools
"DNXT Publisher reduced our submission prep time by 70%. The automated validation catches errors we used to miss in manual review."
"The audit trail and compliance features made our FDA inspection seamless. Everything was documented and easily accessible."
"Multi-tenant architecture means each of our clients has complete data isolation. Security was a key factor in our decision."
Cloud-Native Regulatory Platform
Publish Submissions in Days, Not Weeks
Platform Architecture
Enterprise-Grade, Cloud-Native by Design
Built on Microsoft Azure with a modular, service-oriented architecture. Every component is designed for security, scalability, and seamless integration with your existing regulatory ecosystem. Our Java-based backend delivers platform-independent, deployment-ready operations while a modern frontend ensures fast, engaging experiences across all devices.
Azure Cloud Hosted
21 CFR Part 11
Multi-Tenant SaaS
Open REST APIs
AI-Ready Infrastructure
Modern User Experience
Seamless Integration
Works With Your Existing Stack
No-cost integrations. Connect DnXT to the systems you already use — adoption is smooth and hassle-free.
Built for Compliance
Regulatory-Grade Security & Standards
Every feature is built with life sciences compliance requirements at its core — not bolted on as an afterthought.
01
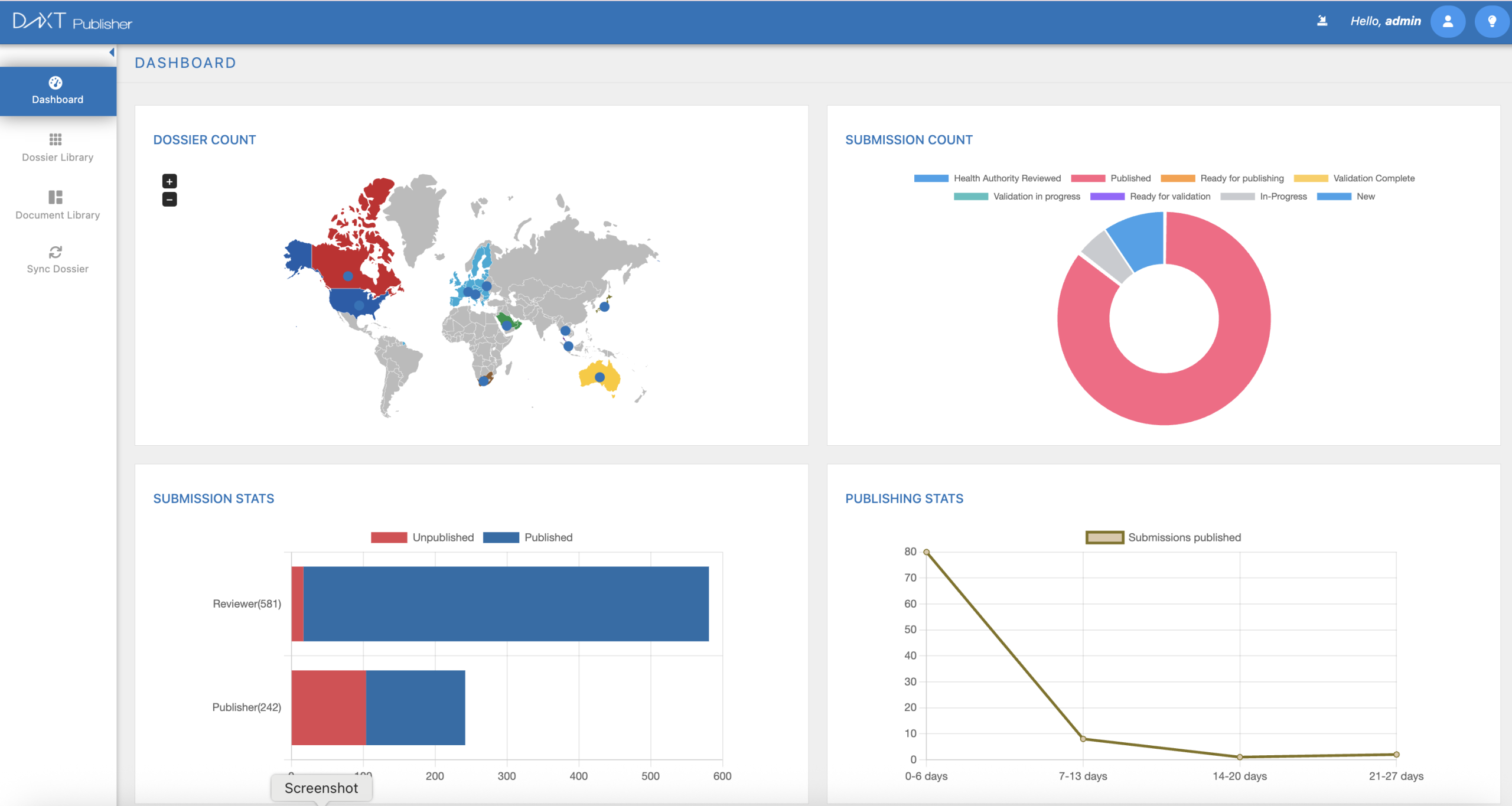
DnXT Publisher
Smarter, Faster eCTD Publishing
Transform complex dossier assembly into a streamlined, automated process. DnXT Publisher delivers compliant, submission-ready eCTD packages with real-time validation, automated metadata management, and direct DMS integration — so your team can focus on strategy, not formatting.
Automated Assembly
Drag-and-drop dossier building with intelligent document ordering and lifecycle management
DMS Integration
Seamless connection to Veeva Vault, SharePoint, and other document management systems
Real-Time Validation
Catch errors before submission with built-in eCTD validation against all regional specifications
Multi-Region Support
Publish to FDA, EMA, Health Canada, TGA, and more from a single platform
02
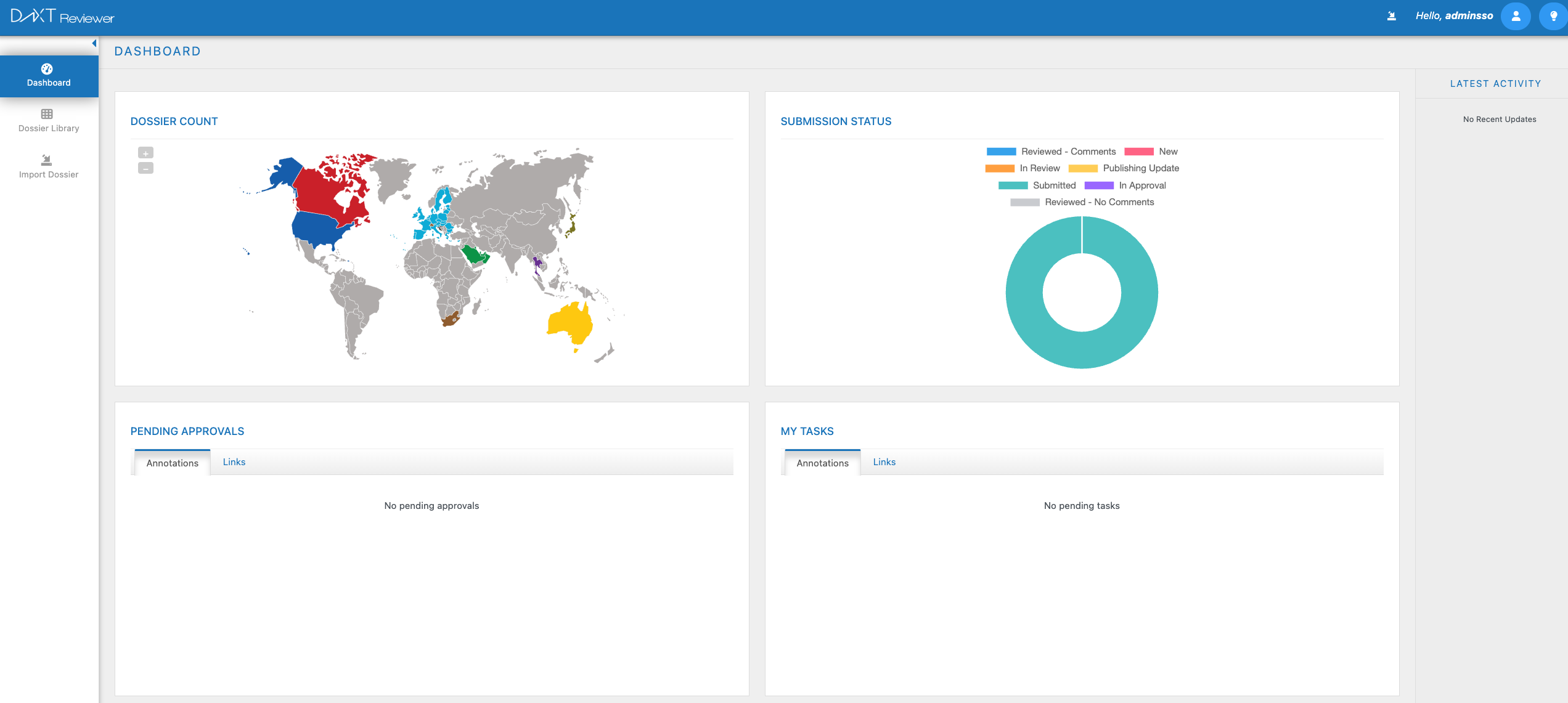
DnXT Reviewer
Redefining eCTD Review Excellence
Navigate complex eCTD submissions with an intuitive, cloud-native review experience. Compare sequences in seconds, collaborate securely across teams, and maintain complete audit trails — all within a single, unified platform built for regulatory professionals.
Lifecycle Tracking
Intelligent eCTD viewing with full lifecycle history and cross-reference navigation
Secure Collaboration
Annotation, comments, and review workflows with role-based access control
Sequence Comparison
Rapid side-by-side comparison of submission sequences with change highlighting
Labeling Comparison
Direct comparison of labeling content across previous lifecycles for faster QC

03
DnXT RIM Suite
Regulatory as a Strategic Capability
A comprehensive Regulatory Information Management suite that treats regulatory operations as a strategic asset, not administrative overhead. Plan submissions, track registrations, manage correspondence, and orchestrate your entire regulatory lifecycle from a single command center.
Submission Planning
Visual timeline management with milestone tracking, dependencies, and agency deadline monitoring
Correspondence Management
Centralized tracking of agency communications with automated response workflows
Registration Tracking
Global registration status dashboard with country-level visibility and renewal alerts
Marketplace Integration
Seamlessly connect with CROs, affiliates, and external partners for controlled outsourcing

04
DnXT AI
Intelligence That Understands Regulation
Purpose-built AI that seamlessly integrates into every stage of the regulatory lifecycle. From automated document classification and real-time validation to predictive compliance alerts and contextual review assistance — DnXT AI transforms manual effort into digital intelligence.
Document Classification
AI-driven tagging, metadata extraction, and automatic CTD section mapping
Compliance Prediction
Early alerts on regulatory changes, risk impacts, and potential submission gaps
Content Summarization
Contextual summaries of submission documents and sequence changes for faster review
Correspondence Analysis
Automated parsing and classification of agency correspondence with action item extraction
Platform Capabilities
Explore Every Feature of DNXT Publisher Suite
Each capability is built for regulatory teams who need speed, compliance, and control — from first draft to agency submission.
WE ARE DnXT
ABOUT US
DnXT Solutions was founded on a simple conviction: regulatory operations should accelerate drug approvals, not slow them down. Our team combines deep regulatory domain expertise with modern software engineering to deliver a platform that regulatory professionals actually want to use. With 340+ submissions published and counting, we are a trusted partner for biotechs, CROs, and pharmaceutical companies navigating the complexities of global regulatory compliance.

CONTACTS
Let's work in your vision
We would be delighted to assist you! Whether you are seeking a demo, an evaluation, or simply want to learn more about how our regulatory publishing and strategy services can benefit your organization, please contact us for a complimentary consultation. We are committed to helping you navigate the regulatory landscape with confidence and efficiency.

