Eliminate eCTD review fatigue with DnXT Reviewer. Fast navigation, side-by-side comparison, and collaborative annotations in one viewer.
Blogs
- DnXT can get your Regulatory Operation process and system tuned up
- DnXT Reviewer and it’s core benefit over other Viewer/ Archive / Review systems
- Eliminating the Link Burden: How DnXT Is Redefining Hyperlink Automation for Regulatory Submissions
- Power of DnXT Platform
- Rendering Perfect PDFs: How DnXT Is Transforming Regulatory Publishing Efficiency
- Why Choose DnXT Regulatory Suite
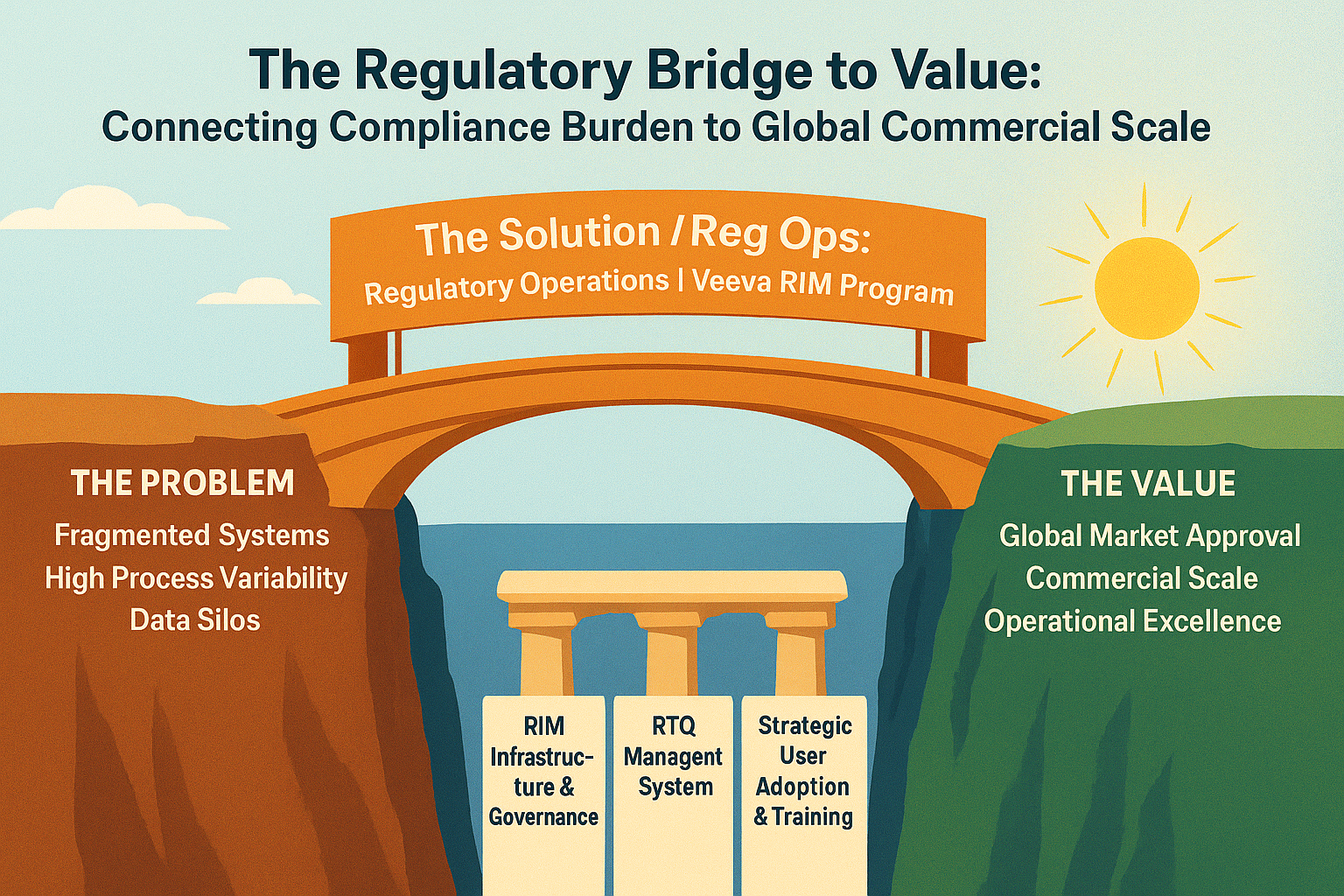
Move beyond compliance to strategic regulatory operations. Scalability, data quality, and submission intelligence insights.
How AI is transforming regulatory affairs from document management to intelligent compliance. Enterprise authoring and analysis at scale.
The Hidden Cost of Inefficient Regulatory Planning Every day pharmaceutical companies lose valuable time and resources to fragmented submission planning processes. Missed deadlines, disconnected teams, and manual tracking systems create bottlenecks that can delay critical drug approvals by months. The financial impact? Potentially millions in lost revenue and extended time-to-market. A Streamlined Solution Built for […]
How DnXT eliminates manual hyperlink creation in regulatory submissions. Automate link generation across your eCTD dossier.
eCTD 4.0 moves to HL7 RPS — a fundamental shift. Here’s what regulatory teams at emerging biotech need to prepare for and what to ask your vendor.
Enterprise RIM pricing goes far beyond the license fee. Here’s the full cost picture for growing biotech — and what to look for in alternatives.
How DnXT transforms PDF rendering for regulatory publishing. Pixel-perfect compliance documents at scale, eliminating formatting issues and manual rework.