While climate change threatens their future, science shows that corals can adapt and survive if we keep them healthy.
Blogs
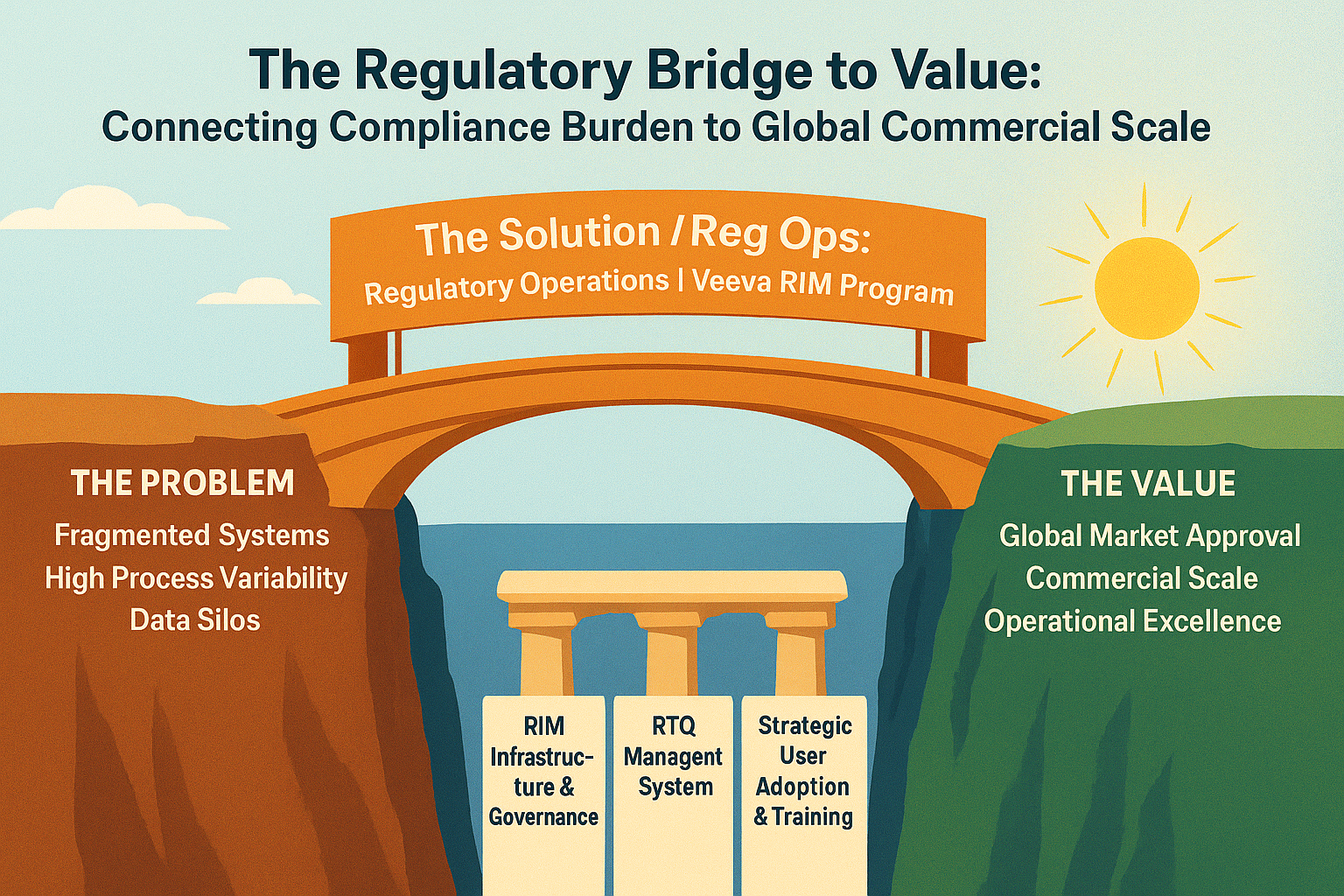
- DnXT can get your Regulatory Operation process and system tuned up
- DnXT Reviewer and it’s core benefit over other Viewer/ Archive / Review systems
- Eliminating the Link Burden: How DnXT Is Redefining Hyperlink Automation for Regulatory Submissions
- Power of DnXT Platform
- Rendering Perfect PDFs: How DnXT Is Transforming Regulatory Publishing Efficiency
- Why Choose DnXT Regulatory Suite
The Hidden Cost of Inefficient Regulatory Planning Every day pharmaceutical companies lose valuable time and resources to fragmented submission planning processes. Missed deadlines, disconnected teams, and manual tracking systems create bottlenecks that can delay critical drug approvals by months. The financial impact? Potentially millions in lost revenue and extended time-to-market. A Streamlined Solution Built for […]
📊 New Report: Industry benchmarks for regulatory operations teams. Download Free → DNXT SOLUTIONS 📄 Free Industry Report • 24 Pages 2025 State of Regulatory Operations The definitive guide for Directors and Managers of Regulatory Affairs. Discover how leading teams are transforming operations, reducing costs, and accelerating submissions. 73% Report resource constraints $2.3M Cost per […]
Top 10 Reasons to go with our eCTD (Electronic Common Technical Document) solution. Several important factors come into play. The relative importance of these factors can vary depending on the specific needs and priorities of our customers, but here are some of the most crucial aspects to consider DnXT’s solution: Compliance with Regulatory Standards: We […]